|
|
| |
|
|
| |
|
|
|
|
| |
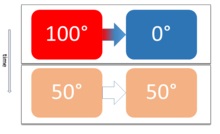 |
| Development of a
thermal equilibrium in a closed system over time
through a heat flow that levels out temperature
differences. |
Equilibrium
In a chemical reaction, chemical equilibrium is the
state in which both reactants and products are present
in concentrations which have no further tendency to
change with time, so that there is no observable change
in the properties of the system. This state results when
the forward reaction proceeds at the same rate as the
reverse reaction. The reaction rates of the forward and
backward reactions are generally not zero, but equal.
Thus, there are no net changes in the concentrations of
the reactants and products. Such a state is known as
dynamic equilibrium.
An equilibrium is a state of a system where all forces
acting on the system is balanced. A system that is in
equilibrium does not change. The word has been used for
different concepts from different fields of study. |
- Hydrostatic equilibrium applies to
liquids.
- Thermal equilibrium means as much
heat is entering and leaving something.
- Homeostasis is a living thing
keeping its internal balance.
- Equilibrioception is how an animal
stands upright.
|
|
Chemical equilibrium
Chemical equilibrium is an idea which describes the
behavior of chemical reactions over time. In reactions
that have finished reacting and have attained equlibrium,
the concentrations of the reactant and product chemicals
do not change.
This means that the forward reaction and the backward
reaction can happen, usually due to a small enthalpy
change in each reaction. Hence, the amount of product
chemicals to reactant chemicals remains a fixed value,
and such a ratio is called the equilibrium constant (Kc). |
|
Equilibrium constant
The equilibrium constant is a ratio of the concentration
of the products raised to the appropriate powers to the
concentration of the reactants raised to the appropriate
powers.
For some reactions, the equilibrium constant is very
large, in which case nearly all of the reactant chemical
is turned into products, such as when paper is burned.
For other reactions, it is very small, which results in
very little products being formed. If the equilibrium
constant is close to 1, a mixture of both products and
reactants is expected at equilibrium.
When chemists talk about equilibrium constants, they
call reactions with large K values favorable and
reactions with small K values unfavorable. It is
important to note, however, these terms are not
judgements about the value or usefulness of a particular
chemical reaction. In fact, some very important
reactions are actually "unfavorable"; one such example
is the synthesis of ammonia from nitrogen and hydrogen
gas (Haber process), which has a rather small
equilibrium constant.
Understanding how chemical equilibriums work is
important as it helps in understanding how a reaction
happens. It allows chemists to calculate how much
products will be formed from a reaction. |
|
Le Chatelier's principle
The concept of Le Chatelier's principle is important in
predicting how an equilibrium will change when different
factors are changed in a system. For example, using the
Le Chatelier's principle, we are able to predict how the
position of equilibrium will change when the
concentration of a certain reactant/product has changed,
as the system will try to counteract the change by
favoring either the forward or the backward reaction |
|
 Kiddle: Equilibrium Kiddle: Equilibrium
Wikipedia: Equilibrium |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Search Fun Easy English |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
About
Contact
Copyright
Resources
Site Map |