|
|
| |
|
|
| |
|
|
|
|
| |
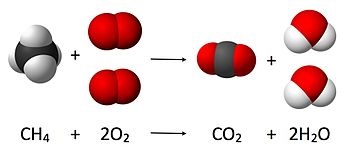 |
| Combustion reaction
of methane. Where 4 atoms of hydrogen, 4 atoms
of oxygen and 1 of carbon are present before and
after the reaction. The total mass after the
reaction is the same as before the reaction. |
Chemical Laws
Chemical laws are those laws of nature relevant to
chemistry. The most fundamental concept in chemistry is
the law of conservation of mass, which states that there
is no detectable change in the quantity of matter during
an ordinary chemical reaction. Modern physics shows that
it is actually energy that is conserved, and that energy
and mass are related; a concept which becomes important
in nuclear chemistry. Conservation of energy leads to
the important concepts of equilibrium, thermodynamics,
and kinetics.
The laws of stoichiometry, that is, the gravimetric
proportions by which chemical elements participate in
chemical reactions, elaborate on the law of conservation
of mass. Joseph Proust's law of definite composition
says that pure chemicals are composed of elements in a
definite formulation; we now know that the structural
arrangement of these elements is also important. |
|
Dalton's law of multiple proportions says that these
chemicals will present themselves in proportions that
are small whole numbers (i.e. 1:2 O:H in water);
although in many systems (notably biomacromolecules and
minerals) the ratios tend to require large numbers, and
are frequently represented as a fraction. Such compounds
are known as non-stoichiometric compounds.
The third stoichiometric law is the law of reciprocal
proportions, which provides the basis for establishing
equivalent weights for each chemical element. Elemental
equivalent weights can then be used to derive atomic
weights for each element. |
|
|
More modern laws of chemistry define the relationship
between energy and transformations. |
- In equilibrium, molecules exist in
mixture defined by the transformations possible on the
timescale of the equilibrium, and are in a ratio defined
by the intrinsic energy of the molecules—the lower the
intrinsic energy, the more abundant the molecule.
- Transforming one structure to
another requires the input of energy to cross an energy
barrier; this can come from the intrinsic energy of the
molecules themselves, or from an external source which
will generally accelerate transformations. The higher
the energy barrier, the slower the transformation
occurs.
- There is a hypothetical
intermediate, or transition structure, that corresponds
to the structure at the top of the energy barrier. The
Hammond-Leffler Postulate states that this structure
looks most similar to the product or starting material
which has intrinsic energy closest to that of the energy
barrier. Stabilizing this hypothetical intermediate
through chemical interaction is one way to achieve
catalysis.
- All chemical processes are
reversible (law of microscopic reversibility) although
some processes have such an energy bias, they are
essentially irreversible.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Search Fun Easy English |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
About
Contact
Copyright
Resources
Site Map |