|
|
| |
|
|
| |
|
|
|
|
| |
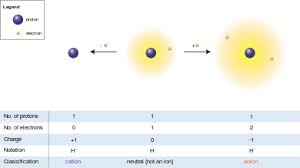 |
| Hydrogen atom
(center) contains a single proton and a single
electron. Removal of the electron gives a cation
(left), whereas addition of an electron gives an
anion (right). The hydrogen anion, with its
loosely held two-electron cloud, has a larger
radius than the neutral atom, which in turn is
much larger than the bare proton of the cation.
Hydrogen forms the only charge-+1 cation that
has no electrons, but even cations that (unlike
hydrogen) still retain one or more electrons are
still smaller than the neutral atoms or
molecules from which they are derived. |
Ions
An ion is an electrically charged atom or group of
atoms. It is a part of an atom, or part of a group of
atoms (molecule). It is "charged" so it will move near
electricity. This is because atoms are made of three
smaller parts (1) neutrons (with no charge), and equal
numbers of (2) charged protons and (3)
oppositely-charged electrons. An ion has unequal numbers
of protons and electrons. Making an ion from an atom or
molecule is called ionization.
The charge on a proton is measured as +1 (positively
charged), and the charge on an electron is measured as
-1 (negatively charged). An atom that is ionized makes
two ions, one positive, and one negatively charged. For
example, a neutral hydrogen atom has one proton and one
electron. Heating the atom breaks it into two parts (1)
a positively charged hydrogen ion, H+ (2) a negatively
charged electron.
A liquid with ions is called an electrolyte. A gas with
lots of ions is called a plasma. When ions move, it is
called electricity. For example, in a wire, the metal
ions do not move, but the electrons move as electricity.
A positive ion and a negative ion will move together.
Two ions of the same charge will move apart. When ions
move they also make magnetic fields. |
|
|
|
|
|
Chemistry
In chemistry, a molecule or atom that is electrically
charged is called an ion. An ion has more or less
electrons than there are protons in an atom. The process
of giving or taking electrons away from a normal atom
and turning it into an ion is called ionization.
Many ions are colourless. Elements in the main groups in
the Periodic Table form colourless ions. Some ions are
coloured. The transition metals usually form coloured
ions. |
|
Physics
In physics, atomic nuclei that have been completely
ionized are called charged particles. These are ones in
alpha radiation.
Ionization happens by giving atoms high energy. This is
done using electrical voltage or by high-energy ionizing
radiation or high temperature.
An ionized gas is called plasma.
A simple ion is formed from a single atom.
Polyatomic ions are formed from a lot of atoms.
Polyatomic ions usually consist of all non-metal atoms,
but sometimes the polyatomic ion can have a metallic
atom too.
Positive ions are called cations. They are attracted to
cathodes (negatively charged electrodes). (Cation is
pronounced "cat eye on", not "kay shun".) All simple
metal ions are cations.
Negative ions are called anions. They are attracted to
anodes (positively charged electrodes). All simple
non-metal ions (except H+, which is a proton) are anions
(except NH4+).
Transition metals can form more than one simple cation
with different charges.
Most ions have a charge of less than 4, but some can
have higher charges.
Michael Faraday was the first person to write a theory
about ions, in 1830. In his theory, he said what the
portions of molecules were like that moved to anions or
cations. Svante August Arrhenius showed how this
happened. He wrote this in his doctoral dissertation in
1884 (University of Uppsala). The university did not
accept his theory at first (he only just passed his
degree). But in 1903, he won the Nobel Prize in
Chemistry for the same idea.
In Greek ion is like the word "go". "Anion" and "cation"
mean "up-goer" and "down-goer". "Anode" and "cathode"
are "way up" and "way down". |
|
Other meanings
In Greek mythology, Ion was a son of Xuthus and Creusa.
He founded the Ionian race and became a king of Athens.
The term is also used for an element of the Plato texts,
and a Window manager. |
|
 Kiddle: Ions Kiddle: Ions
Wikipedia: Ions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Search Fun Easy English |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
About
Contact
Copyright
Resources
Site Map |