|
|
| |
|
|
| |
|
|
|
|
| |
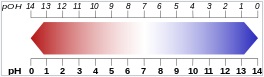 |
| Relation between
p[OH] and p[H] (red = acidic region, blue =
basic region). |
PH
pH (potential of hydrogen) is a scale of acidity from 0
to 14. It tells how acidic or alkaline a substance is.
More acidic solutions, have lower pH. More alkaline
solutions, have higher pH. Substances that aren't acidic
or alkaline (that is, neutral solutions) usually have a
pH of 7. Acids have a pH that is less than 7. Alkalis
have a pH that is greater than 7.
pH is a measure of the concentration of protons (H+) in
a solution. S.P.L. Sørensen introduced this concept in
the year 1909. The p stands for the German potenz,
meaning power or concentration, and the H for the
hydrogen ion (H+).
Alkaline substances have, instead of hydrogen ions, a
concentration of hydroxide ions (OH-). |
|
 |
| Test tubes
containing solutions of pH 1–10 colored with an
indicator. |
pH indicators
A pH indicator is a chemical compound added in small
amounts to a solution so the pH (acidity or basicity) of
the solution can be seen. The pH indicator is a chemical
detector for hydronium ions (H3O+) or hydrogen ions
(H+). Normally, the indicator causes the colour of the
solution to change depending on the pH.
Typical indicators are phenolphthalein, methyl orange,
methyl red, bromothymol blue, and thymol blue. They each
change colour at different points on the pH scale, and
can be used together as a universal indicator.
Another way is to use litmus paper, which is based on a
natural pH indicators. The paper can tell you how strong
the chemical is, whether it is a stronger acid or a
stronger base. |
|
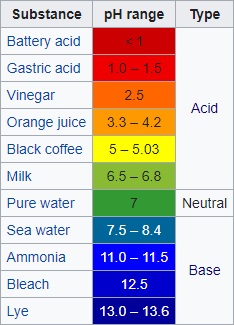 |
| Average pH of common
solutions. |
Some common pH values
Battery acid 1.0
Gastric acid 2.0
Lemon juice 2.4
Cola 2.5
Oxygenated water 2.5 - 3.0
Vinegar 3.0
Orange or apple juice 3.5
Beer 4.5
Coffee 5.0
Milk 6.6
Pure water 7.0
Blood 7.35 - 7.45
Plain shampoo 8.0
Sea water 8.0
Permanent wave 8.5 - 9.2
Hand soap 9.0 - 10.0
Hair dye 9.5 - 10.5
Magic straight 11.5
Household ammonia 11.5
Bleach 12.3
Caustic soda 12.7
Household lye 13.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Search Fun Easy English |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
About
Contact
Copyright
Resources
Site Map |