|
|
| |
|
|
| |
|
|
|
|
| |
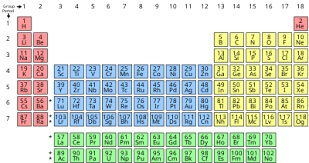 |
| The periodic table
of the chemical elements. |
Periodic Table
The table of chemical elements is a list of known
chemical elements. In the table, the elements are placed
in the order of their atomic numbers starting with the
lowest number of one, hydrogen. The atomic number of an
element is the same as the number of protons in that
particular nucleus of an atom. In the periodic table the
elements are arranged into periods and groups. A row of
elements across the table is called a period. Each
period has a number; from 1 to 8. Period 1 has only 2
elements in it: hydrogen and helium. Period 2 and Period
3 both have 8 elements. Other periods are longer.
Elements in a period have consecutive atomic numbers.
A column of elements down the table is called a group.
There are 18 groups in the standard periodic table. Each
group has a number: from 1 to 18. Elements in a group
have electrons arranged in similar ways, according to
the number of valency electrons, which gives them
similar chemical properties (they behave in similar
ways). For example, group 18 is known as the noble gases
because they are all gases and they do not combine with
other atoms. |
|
|
There are two systems of group numbers; one using Arabic
numerals (1,2,3) and the other using Roman numerals (I,
II, III). The Roman numeral names were used in most of
the 20th century. In 1990 the International Union of
Pure and Applied Chemistry (IUPAC) decided to use the
new system with Arabic numerals, to replace the two old
group systems that used Roman numerals. |
|
|
The periodic table has been used by chemists to observe
patterns and relationships between elements. There are 3
main groups in the Periodic Table; metals, metalloids,
and nonmetals. For example, elements to the bottom and
far left of the table are the most metallic, and
elements on the top right are the least metallic. (e.g.
cesium is much more metallic than helium). There are
also many other patterns and relationships. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Search Fun Easy English |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
About
Contact
Copyright
Resources
Site Map |